Evaporative Cooling
-
Water is naturally one of the fastest heat-absorbing fluids and therefore has a high volatility. When a liquid evaporates, it absorbs heat from its surrounding. When water contacts with air, it absorbs heat from the air and cools the air.
-
Evaporative cooling is based on cooling the air by contacting it with the highest amount of water (wet surface) as much as possible.
-
The coolness we feel in our body when we leave the pool in breezy weather or the coolness we feel when we pour cologne and shake our hands are the best examples of evaporative cooling.
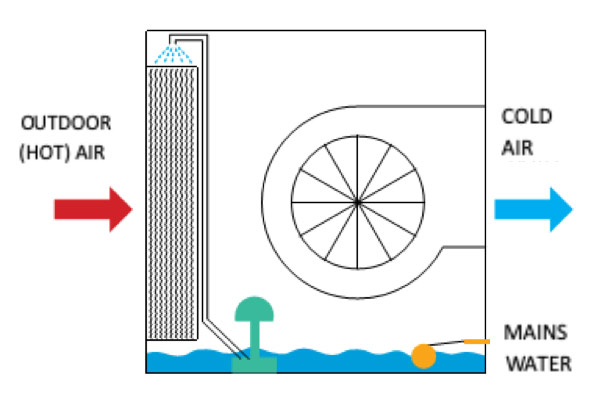
Working Principle
Water is distributed by a small circulation pump in the water reservoir of the device over the cellulosic pads, which thereby are kept always wet. A fan inside the unit absorbs hot air from outside and passes it over these wet pads, and the air passing through the wet pads get cold and is supplied into the room.
It is used commonly for cooling the open and semi-open sections of factories, high ceiling volumes, cafeterias and restaurants and for ventilation with 100% fresh air.